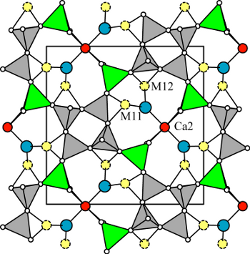
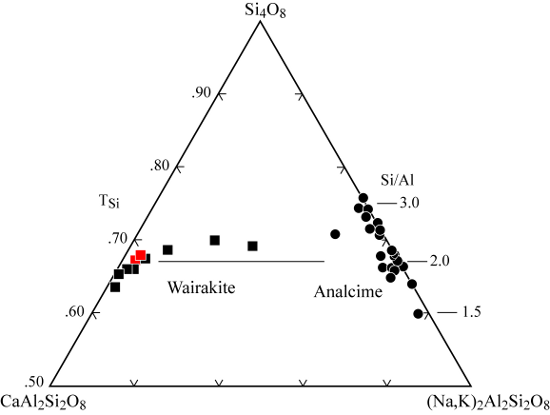
| |
Browne, P.R.L. and Ellis A.J. 1970. The Ohaki-Broadlands hydrothermal area, New Zealand; mineralogy and related geochemistry. Am. J. Sci., 269, 97-131.
Cho, M., Liou, J.G., and Maruyama, S. 1986. Transition from zeolite to prehnite-pumpellyite facies in the Kartmutsen metabasites, Vancouver Island, British Columbia. J. Petr., 27, 467-494.
Coombs, D.S. 1955. X-ray observations of wairakite and non-cubic analcime. Min. Mag. 30, 699-708.
Donnelly, T.W. 1962. Wairakite in West Indian spilitic rocks. Am. Miner., 47, 794-802.
Egeberg, P.K. 1992. Thermodynamic aspects of Leg 126 interstitial waters. Proc. Ocean Drilling Program, Sci. Res., 126, 519-529.
Fiske, R.S., Hopson, C.A., and Waters, A.C. 1963. Geology of Mount Rainier National Park, Washingotn. U.S. Geol. Surv., Prof. Paper 444, 93 pp.
Iijima, A. and Utada, M. 1972. A critical review on the occurrence of zeolites in sedimentary rocks in Japan. Japan. J. Geol. Geogr., 42, 61-83.
Ishizuka, H. 1985. Prograde metamorphism of the Horokanai Ophiolite in the Kamuikotan Zone, Hokkaido, Japan. J. Petrol., 26, 391-417.
Liou, J.G. 1970. Synthesis and stability relations of wairakite, CaAl2Si4O12.2H2O. Contr. Min. Petr., 27, 259-282.
Marsaglia, K.M. and Tazaki, K. 1992. Diagenetic trends in Leg 126 sandstones. Proc. Ocean Drilling Program, Sci. Res., 126, 125-138.
Mazzi, F. and Galli, E. 1978. Is each analcime different? Am. Mineral. 63, 448-460.
Seki, Y. 1966. Wairakite in Japan (I) and (II). Jour. Japan. Assoc. Min. Petr. Econ. Geol., 55, 254-261 and 56, 30-39.
Seki, Y. 1973. Distribution and modes of occurrence of wairakites in the Japanese Island arc. Jour. Geol. Soc. Japan 79, 521-527.
Seki, Y., Liou, J.G., Guillemette, R., Sakai, H., Oki, Y., Hirano, T., and Onuki, H. 1983. Investigation of geothermal systems in Japan I. Onikobe geothermal area. Hydrosci. and Geothechnol. Lab., Saitama Univ., Mem. 3.
Seki, Y., Oki, Y., Matsuda, T., Mikami, K, and Okumura, K. 1969. Metamorphism in the Tanzawa Mountains, Central Japan. Jour. Japan. Assoc. Min. Pet. Econ. Geol., 61, 1-75.
Schiffman, P., Elders, W.A., Williams, A.E., McDowell, S.D., and Bird, D.K. 1984. Active metasomatism in the Cerro Prieto geothermal system, Baja California, Mexico: A telescoped low-pressure, low-temperature metamorphic facies series. Geology, 12, 12-15.
Stakes, D.S. and Schiffman, P. 1999. Hydrothermal alteration within the basement of the sedimented ridge environment of Middle Valley, northern Juan de Fuca Ridge. Geol. Soc. Am., Bull., 111, 1294-1314.
Steiner, A. 1955. Wairakite, the calcium analogue of analcime, a new zeolite mineral. Min. Mag. 30, 691-698.
Surdam, R.C. 1973. Low-grade metamorphism of tuffaceous rocks in the Karmutsen Group, Vancouver Island, British Columbia. Geol. Soc. Am. Bull. 84, 1911-1922.
Takéuchi, Y., Mazzi, F., Haga, and Galli, E. 1979. The crystal structure of wairakite. Am. Mineral. 64, 993-1001.
Utada, M. 1971. Zeolitic zoning of the Neogene pyroclastic rocks in Japan. Sci. Pap. Coll. Gen. Educ. Uni. Tokyo. 21, 189-221.
Utada, M. 2001. Zeolites in hydrothermally altered rocks. In Bish, D.L. and Ming, D.W. (eds) Natural Zeolites: Occurrence, Properties, Applications, Reviews in Mineral. and Geochem., Miner. Soc. Am. 45, 305-322.
Vergara, M., Levi, B., and Villarroel, R. 1993. Geothermal-type alteration in a burial metamorphosed volcanic pile, central Chile. J. Metam. Geol., 11, 449-454.
Vitali, F., Blanc, G., and Larqué,P. 1995. Zeolite distribution in volcaniclastic deep-sea sediments from the Tonga Trench margin (SW Pacific). Clays and Clay Min., 43, 92-104.
Whetten, J.T. 1965. Wairakite from low-grade metamorphic rocks. Am. Mineral., 50, 752-755.
Wise, W.S. 1959. Occurrence of wairakite in metamorphic rocks of the Pacific Northwest. Am. Mineral., 44, 476-480. |