 Flörkeite, Bellerberg volcano near Ettringen, East Eifel volcanic area, Germany. Width of image 1.2 mm. (© Volker Betz)
Flörkeite, Bellerberg volcano near Ettringen, East Eifel volcanic area, Germany. Width of image 1.2 mm. (© Volker Betz)
Hardness: not determined.
D (calc) = 2.266 gm/cm3.
Luster: vitreous.
Streak: white.
Biaxial (-). α = 1.506, β = 1.514, γ = 1.518, δ= 0.012, 2Vx = 70°.
X ˄ c = 43°, Y ˄ b = 40°, Z ˄ b, = 8°
a 19.965, b 14.274, c 8.704 Å , α88.37°, β125.08°, γ 89.57°
Z = 2, Space group P1 (Lengauer et al. 2009).
The framework of flörkeite is the same as that of phillipsite, consisting of chains of doubly connected 4-rings, linked in the UUDD arrangement, generally known as double crankshaft (dcc in PHI). Like in phillipsite the double crankshaft chains run parallel to the a-axis (see PHI), and result in three sets of channels confined by eight-membered rings of tetrahedra, one parallel to the a-axis (shown in the accompanying figure), one parallel to the b-axis, and another parallel to the c-axis. Because of the framework composition (Si/Al = 1.0), there is strict ordering, not only of the framework but also the channel cations. This ordering distorts the phillipsite framework, resulting in the space group P1 (Lengauer et al. 2009).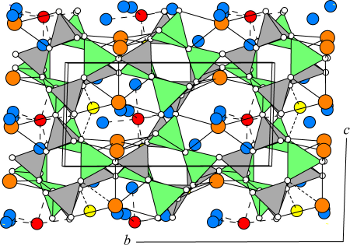
In the diagram Si-tetrahedra are gray, and Al-tetrahedra are green. In the channels K cations (orange) are bonded to four framework oxygens and two to four H2O molecules (blue) in three different sites; Ca cations (red) are bonded to three oxygens and four or five H2O molecules; and Na cations (yellow) are bonded to two oxygens and four H2O molecules.