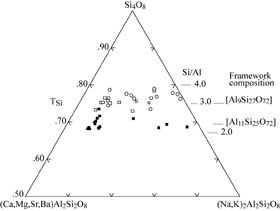
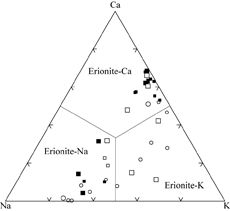
| |
Alberti, A., Martucci, A.,
Galli, E., and Vezzalini, G. (1997) A reexamination of the crystal structure of erionite. Zeolites 19, 349-352.
Anonymous (1986) Asbestos and
Other Natural Mineral Fibres, Environmental Health Criteria 53, IPCS International
Programme on Chemical Safety, World Health Organization, Geneva, 1986. (see
sections 2.2.1 Fibrous zeolites, and specifically 7.2.3 Fibrous zeolites -
erionite (p. 106), and Conclusions, section 9.3.2).
Bargar, K.E. and Keith, T.E.C. (1995) Calcium zeolites in rhyolitic
drill cores from Yellowstone National Park. In:
Ming, D.W. and Mumpton, F.S. (eds.) Natural
Zeolites ’93, International Committee on Natural Zeolites,
Brockport, New York, 69-86.
Chen, N.Y., Maziuk, J., Schwartz, A.B., and Weisz, P.B. (1968) Selectoforming,
a new process to improve octane and quality (of gasoline). Oil
and Gas Journal 66,
154-157.
Chen, N.Y., Lucki, S.J., and Mower, E.B. (1969) Cage effect on product distribution
from cracking over crystalline aluminosilicate zeolites. Journal
of Catalysis 13,
329-332.
Deer, A., Howie, R., Wise, W.S., and Zussman,
J. (2004). Rock Forming Minerals. vol. 4B. Framework
Silicates: Silica Minerals, Feldspathoids and the Zeolites.
The Geological Society, London.
Dogan, A. and Dogan, M. (2008) Re-evaluation and re-classification
of erionite series minerals. Environ. eochem.
Health, 30, 355-366.
Eakle, A.S. (1898) Erionite, a new zeolite. American
Journal of Science 156, 66-68.
Eberly, P.E., Jr. (1964) Adsorption properties of naturally
occurring erionite and its cationic-exchanged forms. American
Mineralogist 49, 30-40.
Gottardi, G. and Galli, E. (1985) Natural Zeolites.
Springer-Verlag, Berlin.
Harada, K., Iwamoto, S., and
Kihara, K. (1967). Erionite, phillipsite and gonnardite in the amygdales
of altered basalt from Mazé, Niigata Prefecture, Japan. American
Mineralogist 52, 1785-1794.
Passaglia, E., Artioli, G., and
Gualtieri, A. (1998) Crystal chemistry of the zeolites erionite and offretite. American
Mineralogist 83, 577-589.
Sheppard, R.A.,
Gude, A.J. III., and Munson, E.L. (1965) Chemical composition of diagenetic
zeolites from tuffaceous rocks of the Mojave Desert and vicinity, California. American
Mineralogist 50, 244-249.
Sheppard, R.A. and Gude, A.J. III (1969) Diagenesis of tuffs in the Barstow
Formation, Mud Hills, San Bernardino County, California. U. S. Geological Survey, Professional
Paper 634, 35 pp.
Sheppard, R.A. and Gude, A.J. III. (1973) Zeolites and
associated authigenic silicate minerals in tuffaceous rocks of the Big
Sandy Formation, Mohave County, Arizona. U.
S. Geological Survey, Professional Paper 830,
36 pp. .
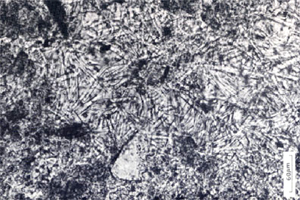
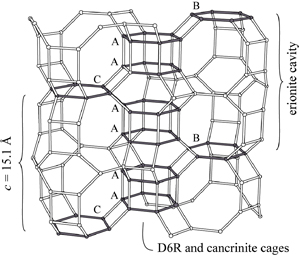
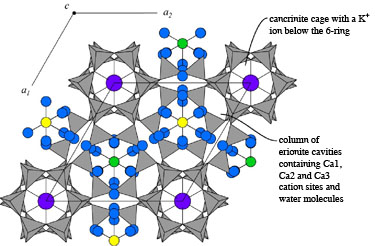
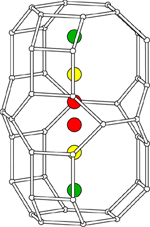
Staples, L.W. and Gard, J.A. (1959) The fibrous zeolite erionite:
its occurrence, unit cell, and structure. Mineralogical
Magazine 32, 261-281.
Tschernich,
R.W. (1992) Zeolites of the World.
Geoscience Press, Phoenix, Arizona, USA. |