Brewsterite-Sr |(Sr,Ba,Ca)2(H2O)10|[Al4 Si12 O32]
Brewsterite-Ba |(Ba,Sr)2(H2O)10|[Al4 Si12 O32]

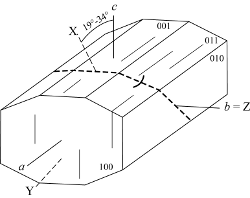
Z = b, X ˄ c = 19° - 34°
Z = b, X ˄ c = 36°
a 6.793, b 17.573, c 7.759 Å, b 94.54°.
Z = 1, Space group P21/m or P21
or commonly: a 6.782, b 17.510, c 7.740, α 89.90°, β 94.09°, γ 90.10°, space group P1
94.47°Z = 1, Space group P21/m or P21
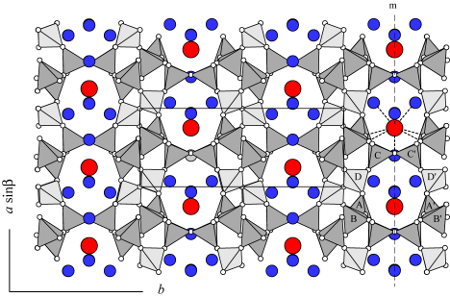
As in all tabular zeolites, there are two sets of interconnecting channels in the framework (see BRE). Eight-membered rings (aperture 2.3 x 5.0 Å) form channels parallel to a-axis, and second set of eight-membered rings (aperture 2.8 x 4.1 Å) forms channels parallel to the c-axis. Schlenker et al. (1977) showed that the framework in the crystal they refined is partially ordered. Almost no Al occurs in D tetrahedral site (light gray tetrahedra), but is approximately evenly distributed between the A, B, and C sites (darker gray tetrahedra), labeled in the lower right corner. Neutron diffraction data by Artioli et al. (1985) confirmed this partial ordering.
Each Sr (or Ba) is bonded to four framework oxygens and five H2O molecules (see the 8-ring channel on the right side of the drawing). Cabella et al. (1993) found that about 92% of Ba occupies the same site as the Sr, but there are two other alternate sites slightly displaced from the Ba1 site, remaining on the mirror plane. Artioli et al. (1985) with neutron diffraction data from a sample of brewsterite from Yellow Lake, British Columbia, showed that the hydrogen atoms for the W4 H2O molecule have three possible orientations. Two of these cause the mirror plane to disappear, lowering the symmetry to P21.
It has been known since the observations of Des Cloizeau (1874) that (010) cleavage sections are divided into three sectors based on extinction angles. These are growth sectors from the {010}, {011}, and {610} faces (Akizuki 1987). Akizuki et al. (1996) show that in a sample from Strontian, Scotland, each sector has a slightly different composition, in both Si/Al and Ba/Sr. Furthermore, each has slightly different Al occupancies in framework tetrahedral sites. Both the {011} and {610} sectors have sufficiently different occupancies between A and A’, B and B’, and C and C’ that they have triclinic symmetry. The occupancies in the {010} sector are very nearly the same across the mirror planes, maintaining monoclinic symmetry.All structure refinements located only one fully occupied channel-cation position. This site is located in the middle of the [100] channels and is nine-coordinated to four framework oxygens and five channel H2O molecules. Artioli et al. (1985) also located the H positions in the channels based on neutron diffraction data. Upon dehydration up to 684 K, brewsterite loses eight of its ten H2O molecules accompanied by diffusion of the channel cations and framework distortion (Ståhl and Hanson 1999). In a dehydration experiment with 24-h equilibration in vacuum at 550 K, Alberti et al. (1999) observed statistical breaking of T-O-T bonds and formation of an altered tetrahedral topology.