| |
|
| Amicite |
|K4Na4 (H2O)10| [Al8Si8O32] |
|
| |
|
|
|
| Morphology: |
|

Amicite crystals from the Kirovsky Mine, Kukisvumcher Mtn., Khibiny Massif, Kola Pennisula, Russia. Width of image is 9 mm. © Volker Betz |
| |
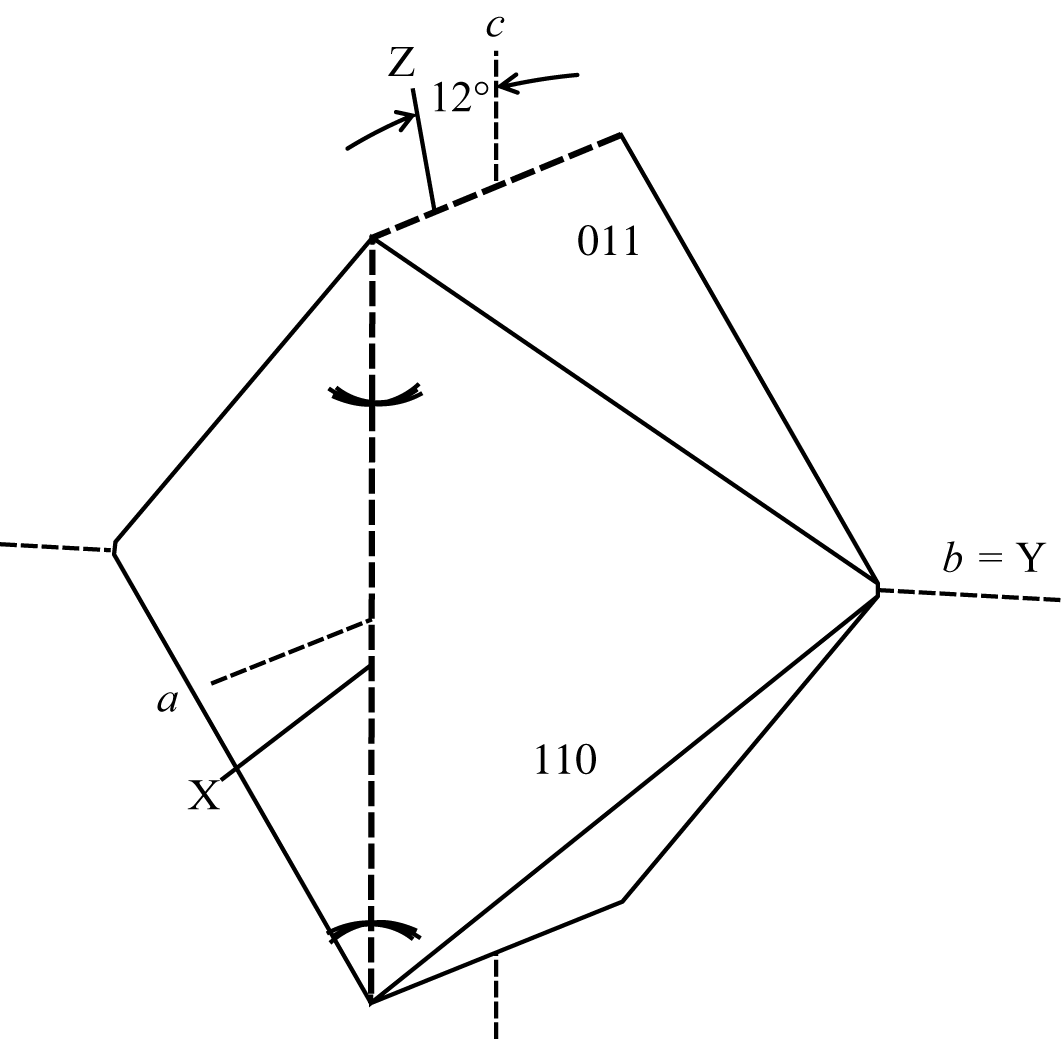
Crystals are bipyramidal pseudotetragonal individuals, to 5 mm., with the forms {011} and {110}. Twinning on {011} and {110} common. |
| |
| Physical
properties: |
| |
Cleavage: none
Fracture: uneven to subconchoidal.
Hardness: 4.5.
D = 2.06 - 2.23 gm/cm3.
Luster: vitreous.
Streak: white. |
| |
|
| Optical properties: |
|
| |
Color: colorless, white to grayish; colorless in thin section. Biaxial (-). α = 1.485, β = 1.490 , γ = 1.494 , δ= 0.009, 2Vx = 82°. Z ˄ c = 12°, Y = b, O.A.P. _|_ (010). |
 |
| |
| Crystallography: |
| |
Unit cell data: a 10.226, b 10.422, c 9.884 Å, β 88.32°.
Z = 1, Space group I2. (Alberti and Vezzalini 1979). |
| |
| |
| |
|
|
| Name: |
|
| |
Alberti et al. (1979) named amicite after Giovan Battista Amici (1786-1863), inventor of the Amici lens and microscope objectives with hemispherical front lens. Type locality is at Höwenegg (a Tertiary melilite nephelninite volcano), Hegau, southwestern Germany. |
| |
|
|
|
| Crystal structure: |
|
| |
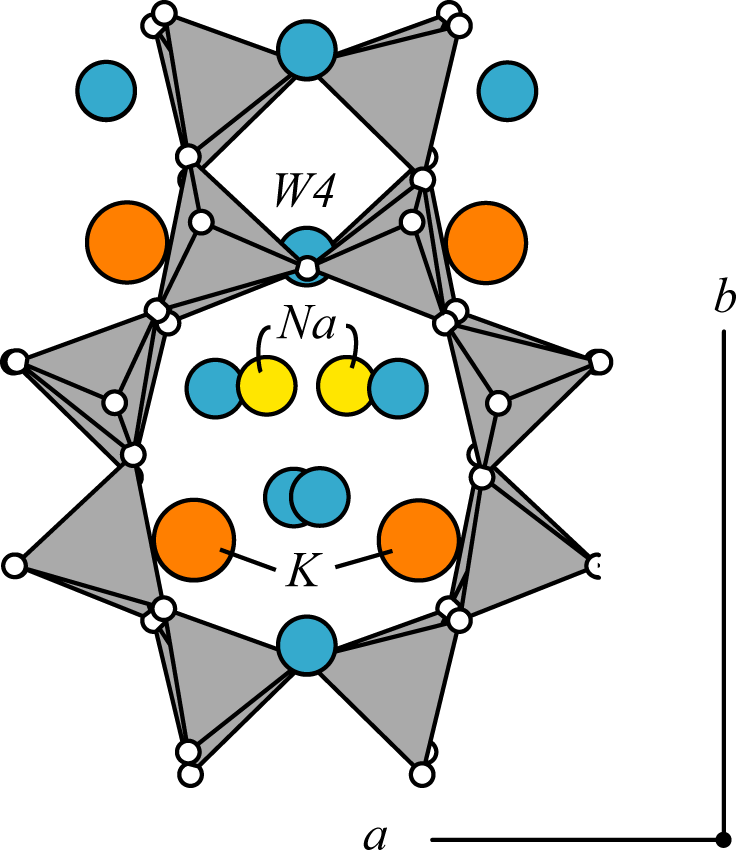
The amicite framework topology consists of two sets of intersecting, doubly connected 4-membered rings linked into double crankshaft chains (dcc on the GIS page). These sets of double crankshaft chains that run parallel to the a-axis and to the b-axis are related by a 41 axis forming the GIS framework. Where there is (Si,Al) disorder in the tetrahredra, the topological symmetry is I41/amd. However, in amicite ordering of the framework and restrictions caused by distribution of channel cations and H2O molecules lower the symmetry to I2. |
| |
Through this framework, channels confined by eight-membered rings (aperture 3.0 x 4.7 Å run parallel to the a- and b-axes. Na (yellow) and K (orange) are well ordered in two completely occupied sites. Na is six-coordinated by three oxygens and three H2O molecules (blue). K is seven-coordinated by four framework oxygens and three H2O molecules. In amicite, two of the gismondine Ca sites are occupied by Na with two additional Na sites. K sites in amicite are occupied in gismondine by H2O. Three filled H2O sites are bonded to channel cations. One additional H2O site is only half occupied and with a long distance (2.9 Å) to Na.
Amicite can be completely dehydrated without destruction of the framework (Vezzalini et al. 1999). |
 |
| Chemical composition: |
| |
There are only two known occurrences of amicite, Hegau, Germany, and Khibiny massif, Russia. The framework composition of these two samples is TSi 0.51 and 0.49, respectively. In both samples Na is approximately equal to K, and Ca is very minor in amount. In both published analyses the H2O content is about 12.9 weight percent corresponding to 10 molecules per unit cell. |
| |
|
| Occurrences: |
| |
At the type locality amicite occurs with merlinoite in veins cutting melilite nephelinite at Höwenegg, Hegau, Germany. In Russia it occurs in natrolite veinlets cutting ijolite-urtite pegmatite and apatite-nepheline rock at the S.M. Kirov Mine, Mt. Kukisvumchorr, in the Khibina massif, Kola Peninsula (Khomyakov et al. 1982). |
| |
|
| References: |
| |
Alberti, A., Hentschel, G. and Vezzalini, G. 1979.
Amicite, a new natural zeolite. Neues Jahrb. Mineral., Monatsh., 1979, 481-488.
Alberti, A. and Vezzalini, G. 1979. The crystal structure of amicite, a zeolite. Acta Crystallogr. B37, 781-788.
Khomyakov, A.P., Cherepivskaya, G.E. and Kurova, T.A. 1982.
Amicchite, K2Na2Al4Si4O16•5H2O, first find in the USSR. Dokl. Acad. Sci. U.S.S.R., Earth Sci. Sect. 263, 135-137.
Vezzalini, G., Alberti, A., Sani, A., and Triscari, M. 1999. The dehydration process in amicite. Microporous Mesoporous Mater. 31, 253-161. |


