Paulingite-K |(K,Ca0.5,Na)10 (H2O)27-34|[Al10Si32O84]

Hardness: 4 - 4.5.
D = 2.085 gm/cm3 (paulingite-Ca)
2.098 gm/cm3 (calc.) ( paulingite-K).
Luster: vitreous.
Streak: white.
Isotropic.
n 1.482 - 1.484 (paulingite-Ca)
n 1.473 - 1.484 (paulingite-K)
The structure of paulingite was determined by Gordon et al. (1966), confirming the space group Im3m, proposed by Kamb and Oke (1960). The unit cell (a = 35.1 Å) is the largest of all known zeolites, and the framework filling this cell is a complex, though elegant, arrangement of cages and double 8-rings (see PAU). The framework consists of four regular polyhedra or cages and three irregular polyhedra, accounting for the interstices. An alpha-cage (also named lta in the drawingsof PAU) is located at the cell center and at the cell corners. Connected to each of the six single 8-ring faces of the lta are d8R (double 8-ring) and pau polyhedra (see PAU). The sequence of these cages is lta- d8R-pau- d8R-pau- d8R-lta along one edge of the unit cell. Each unit cell contains 672 tetrahedral sites, and the Si,Al distribution throughout these sites appears to be random (Bieniok et al. 1996, Lengauer et al. 1997).
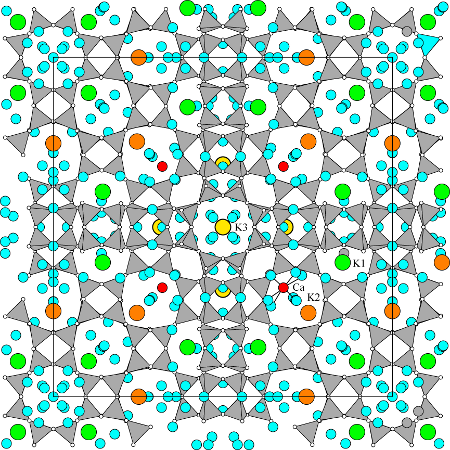
The structure refinement of paulingite-K from Rock Island Dam crystals by Gordon et al. (1966) yielded locations for about 75% of the cations and about 67% of the H2O molecules. More recent refinements by Bieniok et al. (1996) of paulingite-K from Chase Creek, British Columbia and by Bieniok (2000) of paulingite-Ca from Ritter, Oregon, and by Lengauer et al. (1997) of paulingite-Ca from Vinarická Hore, Czech Republic, located more of the 110 to 130 cations and more than 500 H2O molecules per unit cell. Although there is some variation of cation sites from sample to sample, there are also some consistencies. Each of the plg cages contains a site (16 positions per cell) occupied mostly by Ca2+ cations (red in the figure), each surrounded by eight H2O molecules. The K1 site (48 positions per cell, green) is located in each of the irregular 8-ring openings of the pau cage and is coordinated with four framework oxygen anions and two H2O molecules. The K2 sites (24 positions per cell, orange) are outside the plg cages and are generally occupied by K, Ca, and if present, Ba. K2 cations are coordinated with two to six framework oxygen anions, depending on occupancy, and to four H2O molecules. Finally, K3 sites (12 positions per cell, yellow) are within the opr polyhedra connected to grc cages. This site is occupied by K, especially in K-dominant crystals, and is surrounded by six H2O molecules. These four sites account for about 100 cations per cell. The remaining cations, detected by chemical analyses but not located by the refinements, may be randomly distributed among some of the H2O molecule sites not bonded to located cation sites.