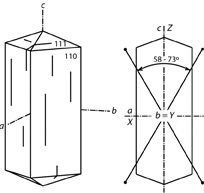
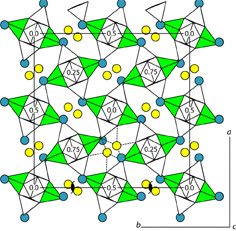
| |
Alberti, A. and Vezzalini,
G. (1981) A partially disordered natrolite: relationships between cell
parameters and Si-Al distribution. Acta Crystallogr. B37,
781-788.
Alberti, A., Cruciani, G., and Dauru, I. (1995) Oder-disorder in natrolite-group
minerals. Eur. J. Mineral. 7,
501-508.
Alberti, A., Pongiluppi, D., and Vezzalini, G. (1982) The crystal chemistry
of natrolite, mesolite and scolecite. Neues
Jahrb. Miner. Monatsh. 1982,
231-248.
Artioli, G., Smith, J.V., and Kvick, A (1984) Neutron diffraction study
of natrolite, Na2Al2Si3O10 2H2O at 20 K. Acta
Crystallogr. C40,
1658-1662.
Barnes, D.A., Boles, J.R., and Hickey, J. (1984) Zeolite occurrences
in Triassic-Jurassic sedimentary rocks, Baja California, Mexico. In Olson,
D. and Bisio, A. (eds.) Proceedings of the
Sixth International Zeolite Conference, Reno, USA., Butterworths,
Guildford, UK, 905-913.
Bass, N.N., Moberley, R., Rhodes, J.M., Shih, C., and Church, S.E. (1973)
Volcanic rocks cored in the Central Pacific, Leg 17, Deep Sea Drilling
Project Initial Rpt. DSDP 17, 429-503.
Birch, W.D. (1989) Chemistry of Victorian zeolites. In Birch, W.D. Zeolites
of Victoria. Min. Soc. Victoria (Australia), Spec. Publ. No. 2,
91-102.
Chao, G.Y. (1980) Paranatrolite, a new zeolite from Mont St-Hilaire,
Quebec. Can. Mineral. 18,
85-88.
Chen, T.T. and Chao, G.Y. (1980) Tetranatrolite from Mont St-Hilaire,
Quebec. Can. Mineral. 18,
77-84.
Coombs, D.S. et al. (1997) Recommended nomenclature for zeolite minerals:
Report of the Subcommittee on Zeolites of the International Mineralogical
Association, Commission on New Minerals and Mineral Names. Can.
Mineral. 35,
1571-1606.
Deer, A., Howie, R., Wise, W.S., and Zussman, J. (2004). Rock
Forming Minerals. vol. 4B. Framework
Silicates: Silica Minerals, Feldspathoids and the Zeolites.
The Geological Society, London.
Flohr, M.J.K. and Ross, M. (1989) Alkaline
rocks of Magnet Cove, Arkansas: metasomatized ijolite xenoliths from Diamond
Jo quarry. Am. Mineral.
74, 113-131.
Foster, M.D. (1965a) Composition
of zeolites of the natrolite group.
U.S. Geol. Surv., Prof. Paper 504-D,
7 pp.
Foster, M.D. (1965b) Compositional relations among thomsonites,
gonnardites, and natrolites. U.S. Geol. Surv.,
Prof. Paper 504-E,
10 pp.
Gnos, E., Mahmood, K., Khan, M., Khan, A. S., and Armbruster,
T. (1999) Natrolite from the Bela Ophiolite, Pakistan. Jour.
Gem. 26,
308-312.
Grice, J.D. and Gault, R.A. (1981) Edingtonite and natrolite
from Ice River, British Columbia. Mineral.
Rec. 12,
221-230.
Gunter, M.E., Knowles, C.R., and Schalck, D.K. (1993) Composite
natrolite-mesolite crystals from the Columbia River Basalt Group, Clarkston,
Washington.
Can. Mineral. 31,
467-470.
Haüy, R.-J. (1801) Traité de minéralogie 3.
Chez Louis, Paris, France.
Hay, R.L. (1966) Zeolites and zeolitic reaction
in sedimentary rocks.
Geol. Soc. Amer., Spec. Pap. 85,
130 pp.
Hay, R.L. (1970) Silicate reactions in three lithofacies
of a semi-arid basin, Olduvai Gorge, Tanzania. Min. Soc. Amer., Spec.
Pap. 3,
237-255.
Hey, M.H. (1932) Studies on the zeolites. Part III. Natrolite
and metanatrolite.
Mineral. Mag. 23, 243-289.
Horváth,
L. and Gault, R.A. (1990) The mineralogy of Mont Saint-Hilaire, Quebec. Mineral.
Rec. 21, 284-359.
Houghton, B.F. (1982) Low-grade metamorphism of the Takitimu
Group, western Southland, New Zealand. New Zeal. J. Geol. Geoph. 25,
1-19.
Ishizuka, H.
(1985) Prograde metamorphism of the Horokanai Ophiolite in the Kamuikotan
Zone, Hokkaido, Japan. J. Petrol. 26,
391-417.
Klaproth, M.H. (1803) Chemische Untersuchung des Natroliths. Ges.
Naturforschender Freunde zu Berlin, Neue Schriften 4,
243-248.
Kristmannsdóttir, H. and Tómasson, J. (1978) Zeolite zones
in geothermal areas in Iceland. in Natural
Zeolites, Occurrence, Properties, Use, Pergamon
Press, Elmsford, N.Y., 277-284.
Krogh
Andersen, E., Danø, M., and Petersen, O.V. (1969) A tetragonal natrolite. Meddr.
om Grønlands 181, 20 pp. [M.A.
74-1451].
Labuntzov, A.N. (1927) The zeolites from Khibinsky and
Lovozersky Mtns., Russian Lapland (in Russian). Trav.
Musée Minér. Acad. Sci. USSR 2,
91-100.
Lee, Y., Vogt, T., Hriljac, J.A., Parise, J.B., and Artioli,
G. (2000) Pressure-induced volume expansion of zeolites in the natrolite
family. J.
Am. Chem. Soc.
124 (19), 5466 -5475.
Louderback,
G.D. (1907) Benitoite, a new California gem mineral. Univ.
Calif. Publ., Bull. Dept. Geol. 5,
149-153.
Mason, B. (1946) Analcite, apophyllite, and natrolite from
the Pahau River, North Canterbury. New Zeal.
Jour. Sci. and Tech. 28, 53-54.
Meier,
W.M. (1960) The crystal structure of natrolite. Z.
Kristallogr.
113, 430-444.
Neuhoff, P.S., Kroeker,
S., Lin-Shu Du, Fridrikson, T., and Stebbins, J. (2002) Order/disorder
in natrolite group zeolites: A 29Si and 27Al MAS
NMR study. Am.
Mineral. 87,
1307-1320.
Pabst, A. (1971) Natrolite from the Green River formation,
Colorado, showing an intergrowth akin to twinning. Am.
Mineral. 56, 676-680.
Panzner,
W.D. (1987) Minerals of Mexico.
van Nostrand Reinhold Co. Inc., New York, 459 pp.
Pauling, L. (1930)
The structure of sodium and calcium aluminosilicates.
Proc. Nat. Acad. Sci. USA 16,
453-459.
Pekov, I.V. (2000) Lovozero Massif:
History, Pegmatites, Minerals. Ocean Pictures
Ltd., Moscow, Russia. 484 pp.
Poitevin, E. (1938) Natrolite
from the eastern townships, Quebec. Univ.
Toronto Studies, Geol. Ser. 41,
57-58.
Ramdohr, P. (1978) Der Natrolith vom Hohentwiel. Lapis 3,
18-19.
Ross, M., Flohr, M.J.K., and Ross, D.R. (1992) Crystalline solution
series and order-disorder within the natrolite mineral group. Am.
Mineral. 77,
685-703.
Sassen, R. (1973) Natrolite--New Jersey. Mineral.
Rec. 4, 55-56.
Sassen, R. 1978. Natrolite and associated secondary
minerals at the Chimney Rock Quarry, Bound Brook, New Jersey. Mineral.
Rec. 9, 25-31.
Senderov,
E.E. and Khitarov, N.I. (1971) Synthesis of thermodynamically stable
zeolites in the Na2O-Al2O3-SiO2-H2O.
In. Molecular
Sieve Zeolites - 1. Amer. Chem. Soc., Adv. Chem. Ser. 101,
149-154.
Taylor, W.H., Meek, C.A., and Jackson, W.W. (1933) The structures
of the fibrous zeolites. Z. Kristallogr. 84,
373-398.
Tschernich, R.W. 1992 Zeolites
of the World,
Geoscience Press, Phoenix, Arizona. 563 pp.
Walker, G.P.L. (1960a)
The amygdale minerals in the Tertiary lavas of Ireland. III. Regional
distribution. Mineral.
Mag. 32, 503-527.
Walker,
G.P.L. (1960b) Zeolite zones and dike distribution in relation to the
structure of the basalts of eastern Iceland. Jour.
Geol. 68, 515-528.
Walker,
G.P.L. (1962) Garronite, a new zeolite, from Ireland and Iceland.
Mineral. Mag. 33,
173-201.
Wise, W.S. and Gill, R.H. (1977) Minerals of the Benitoite
Gem Mine. Mineral.
Rec. 8, 442-452. |