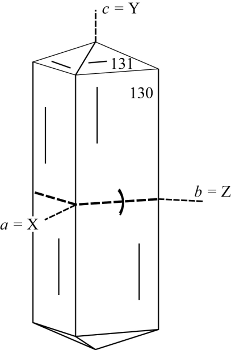
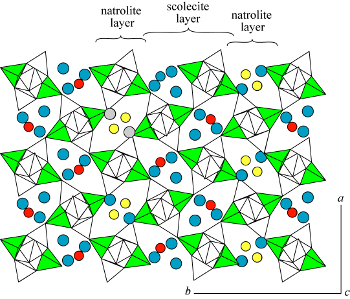
| |
Mesolite has limited occurrences worldwide with most occurring in cavities and fracture of mafic volcanic rocks.
Diagenesis of sediment and sedimentary rocks
Mesolite rarely occurs as an authigenic mineral in sedimentary rocks. Such occurrences tend to involve unusual chemical compositions of the host rocks or interstitial waters.
Diagenesis of marine sediment from arc-source terrains. Jurassic to Cretaceous of the Eugenia Formation exposed on the western Vizcaino Peninsula, Baja California Sur, Mexico, contain authigenic natrolite and mesolite, heulandite-Na, and analcime (Barnes et al. 1984). These zeolites replace plagioclase and glassy vitric fragments, as well as form cement, in the heterogenous marine volcaniclastic rocks.
Diagenesis and very low grade metamorphism of basalt and other kinds of lava flows.
Cavity and vein filling in altered basaltic lavas is the setting for most occurrences of mesolite. The occurrences in Iceland provide relationships that indicate the general temperatures and depths of formation of fibrous zeolites. In eastern Iceland the lowest exposures are rich in zeolites and define a zone from below sea level to about 800 m, which is overlain by the analcime zone (Walker 1960).
Well known mesolite occurrences in basalt include cavities in Tertiary shoshonite flows in the Table Mountains at Golden, Jefferson County, Colorado, where it is closely associated with thomsonite (Kile and Modreski 1988); as 4 cm long needles in altered basalt at the Bear Creek quarry, Drain, Douglas County and in pockets between two basalt flows east of Ritter Hot Springs, Grant County, Oregon; as long prisms as much as 10 cm from cavities in Eocene basalt at Skookumchuck Dam near Tenino, Thurston County, Washington (Tschernich 1972), all in the USA; as fibrous masses in Triassic basalt in the western part of the Bay of Fundy, Nova Scotia, Canada (Walker and Parsons 1922); as 10 cm long crystals in vesicular Tertiary basalt at Naalsoy, Stremoy, Faeroe Islands (Betz 1981); fine fibers with natrolite in vesicular basalt at Neubauerberg, Bohemia, Czech Republic; and as long prisms in the Deccan basalt in the Pune (Poona) district, India (Currier 1976).
Active and fossil hydrothermal systems.
Mesolite (and scolecite) are known only in the Icelandic geothermal areas. Kristmannsdóttir and Tómasson (1978) define Zone 2 as the Mesolite/Scolecite Zone, probably forming in the temperature interval between 70° and 90°C. The controlling factor is the effect that the basaltic walls rocks have on the geothermal fluids from which the zeolites crystallize.
Fossil hydrothermal systems are exposed in eastern part Martinique, French West Indies. Westercamp (1981) describes concentric zoning of alteration around several centers, that suggest hydrothermal alteration around earlier volcanic conduits. Within the outermost zone chabazite, heulandite or clinoptilolite, mordenite, and mesolite are common in cavities and veins of altered lavas. Inward these zeolites are replaced by analcime and thomsonite. |
| |
Adiwidjaja, G. 1972. Struturbeziehungen in der Natrolithgruppe und das Entwässerungsverhalten des Skolezits. Dissertation. Univ. Hamburg.
Alberti, A., Cruciani, G., and Dauru, I. 1995. Order-disorder in natrolite-group minerals. Eur. J. Mineral., 7, 501-508.
Alberti, A., Pongiluppi, D., Vezzalini, G. 1982. The crystal chemistry of natrolite, mesolite and scolecite. Neues Jahrb. Miner. Monatsh. 1982, 231-248.
Barnes, D.A., Boles, J.R., and Hickey, J. 1984. Zeolite occurrences in Triassic-Jurassic sedimentary rocks, Baja California, Mexico. In Olson, D. and Bisio, A. (eds). Proceedings of the Sixth International Zeolite Conference, Reno, USA., Butterworths, 905-913.
Betz, V. 1981. Zeolites from Iceland and the Faeroes. Min. Rec. 12, 5-26.
Currier, R.H. 1976. Production of zeolite mineral specimens from the Deccan Basalt in India. Min. Rec. 7, 248-264.
Deer, A., Howie, R., Wise, W.S., and Zussman, J. (2004). Rock Forming Minerals. vol. 4B.
Framework Silicates: Silica Minerals, Feldspathoids and the Zeolites. The Geological Society, London.
Foster, M.D. 1965a. Composition of zeolites of the natrolite group. U.S. Geol. Surv., Prof. Paper 504-D, 7 pp.
Foster, M.D. 1965b. Compositional relations among thomsonites, gonnardites, and natrolites. U.S. Geol. Surv., Prof. Paper 504-E, 10 pp.
Gehlen, A.F. and Fuchs, J.N. 1813. Über Werner’s Zeolith, Haüy’s Mesotype und Stilbite. (Schweigger’s) J. Chem. und Phys. 8, 353-366.
Gunter, M.E., Knowles, C.R. and Schalck, D.K. 1993. Composite natrolite-mesolite crystals from the Columbia River Basalt Group, Clarkston, Washington. Can. Min. 31, 467-470.
Haüy, R.-J. 1801. Traité de minéralogie 3. Chez Louis, Paris, France.
Hey, M.H. 1932. Studies on the zeolites. Part III. Natrolite and metanatrolite. Min. Mag. 23, 243-289.
Kile, D.E. and Modreski, P.J. 1988. Zeolites and related minerals from the Table Mountain lava flows near Golden Colorado. Min. Rec. 19, 153-184.
Kristmannsdóttir, H. and Tómasson, J. 1978. Zeolite zones in geothermal areas in Iceland. in Natural Zeolites, Occurrence, Properties, Use, Pergamon Press, Oxford., 277-284.
Ross, M., Flohr, M.J.K., and Ross, D.R. 1992. Crystalline solution series and order-disorder within the natrolite mineral group. Am. Mineral. 77, 685-703.
Ståhl, K. and Thomasson, R. 1994. The dehydration and rehydration processes in the natural zeolite mesolite studied by conventional and synchrotron X-ray powder diffraction. Zeolites, 14, 12-17.
Tschernich, R.W. 1992 Zeolites of the World, Geoscience Press, Phoenix, Arizona. 563 pp.
Walker, G.P.L. 1960. Zeolite zones and dike distribution in relation to the structure of the basalts of eastern Iceland. Jour. Geol. 68, 515-528.
Walker, T.L. and Parsons, A.L. 1922. The zeolites of Nova Scotia. Univ. Toronto Studies, Geol. Ser., 16, 13-73.
Westercamp, D. 1981. Distribution and volcano-structural control of zeolites and other amygdale minerals in the Island of Martinique, F.W.I. J. Volc. and Geotherm. Res. 11, 353-365. |